NANORADIOENHANCER PLATFORM

Enabling a new era of treatment for millions of patients with cancer
The vast majority of patients with cancer in need of treatment present with local disease (i.e. a single primary tumor) at diagnosis or have undergone treatment for local disease that has failed. Patients who present with metastatic disease (i.e. tumors that have spread beyond the primary) are a smaller group.
Radiotherapy is the most widely used treatment for patients with local cancer or local and metastatic cancer, treating 12 million new patients around the world each year.1 When used in combination with other therapies like surgery, chemotherapy, or targeted therapies, many patients have positive treatment outcomes. However, despite technological advancements that increase the efficiency of radiation dosing to specific, well-defined areas, damage to normal tissue is unavoidable at the doses required for radiotherapy to be effective. As a result, treatment efficacy from radiotherapy remains limited by the dose that can be administered.2
First-in-class nanoradioenhancer NBTXR3, is designed to increase the dose of radiotherapy that is absorbed and deposited into tumor cells without increasing the dose in surrounding healthy tissues, thereby destroying the target tumor and priming the immune response attack on metastatic tumors. By significantly increasing the tumor-killing effect of radiotherapy without increasing damage to normal tissue, NBTXR3 offers potential to address the unmet needs of millions of patients with cancer.
Seamless integration into the existing treatment landscape
NBTXR3 is designed to significantly increase the energy deposited by radiotherapy within tumor cells to induce significant tumor cell death without increasing damage to surrounding healthy tissues.
NBTXR3 at-a-glance
Administered via one-time injection directly into a malignant tumor prior to standard ionizing radiotherapy
The unique physical properties of NBTXR3 enable more radiation to accumulate locally within cancer cells
Once activated, NBTXR3 increases the energy deposited by radiotherapy within injected tumor cells up to 9 times compared to radiotherapy alone.3 Clinical data suggest that the mechanism of action of NBTXR3 ultimately induces significant tumor cell death without increasing the damage to surrounding healthy tissues.4 Because NBTXR3 nanoparticles return to their inactive state after each exposure to radiation, multiple courses of radiotherapy can be administered to a tumor after it has received a single injection of NBTXR3.
Implications for immuno-oncology
Early clinical data suggest that radiotherapy-activated NBTXR3 may make cancer cells more visible to the immune system and prime the body’s immune response. We believe that this may happen in several ways. One mechanism involves the release of damage-associated molecular patterns that are recognized by immune cells as a sign of cellular stress or injury.5 This may trigger an immune response against both the primary tumor and metastatic cells due to immunogenic cell death, a type of cell death that leads to activation of the immune system. Additionally, NBTXR3 has been shown to increase the expression of cell-surface proteins that enhance the presentation of tumor antigens to immune cells, possibly leading to improved recognition and destruction of cancer cells.6
NBTXR3 in head and neck squamous cell carcinoma (HNSCC)
HNSCC is the sixth most common malignancy worldwide, and as many as 40% of patients with HNSCC are aged 70 years or older. Because these patients are often frail due to multiple comorbidities, they are typically poor candidates for more aggressive treatment regimens.7 Given the urgent need for safer, more effective therapies in these especially vulnerable patients, Nanobiotix chose this cohort to determine the safety and effectiveness of radiotherapy-activated NBTXR3.
In 2019, the US FDA issued Fast Track development designation for the investigation of NBTXR3 in combination with radiotherapy with or without cetuximab for patients with locally advanced HNSCC who were ineligible for platinum-based chemotherapy. In a phase 1/2 study, NBTXR3 was found to improve the tumor response, with a higher proportion of patients achieving a complete response compared with patients who received radiotherapy alone. NBTXR3 was also found to have a favorable safety profile.
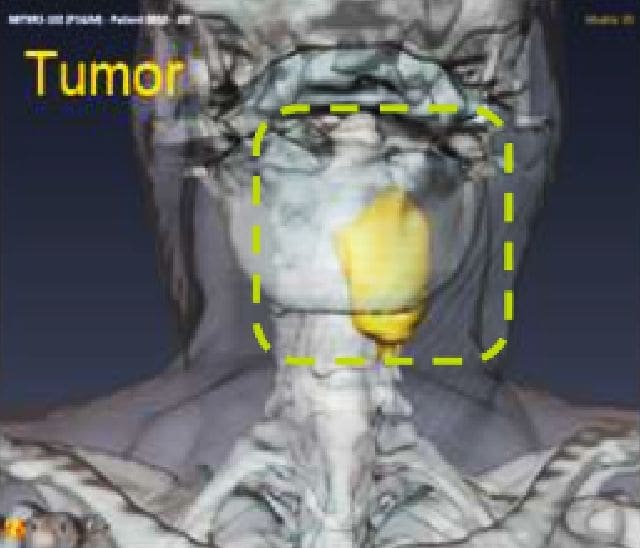
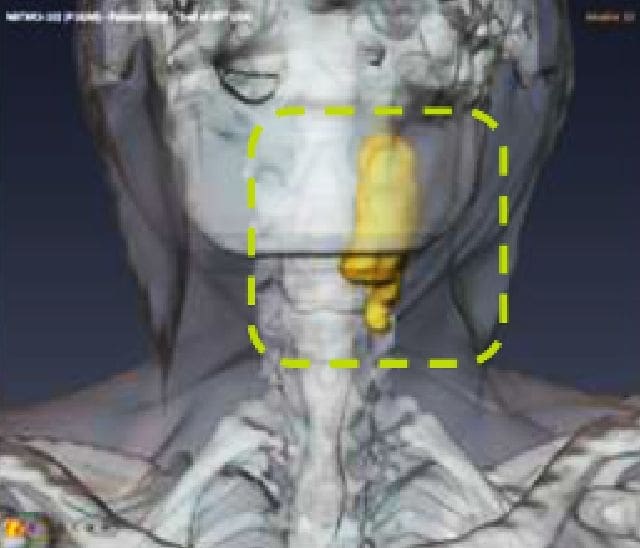
Preliminary positive results from a completed phase 1 dose escalation and dose expansion trial results suggest that NBTXR3 could improve treatment outcomes in patients with HNSCC and has the potential to become an important treatment option, especially for vulnerable patients who are not candidates for other treatments. A phase 3 global registration trial evaluating NBTXR3 in this population is currently under way.
Toward a universal radioenhancer
Since the mechanism of action of NBTXR3 is physics-based rather than biological or chemical, we anticipate that its effects will be scalable across a range of solid tumors and treatment lines. In a phase 2/3 clinical trial involving 176 patients with soft tissue sarcoma, those who received NBTXR3 in combination with radiotherapy had significantly increased rate of pathological complete response than those receiving standard of care radiotherapy alone.8 Combined with early-to-late stage data across several indications including head and neck cancer, liver cancer, and pancreatic cancer, these data suggest that radiotherapy-activated NBTXR3 may offer a new treatment option for an array of solid tumors when used on its own or in combination with other therapies.
References: 1. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and Radiation Therapy: Current Advances and Future Directions. Int J Med Sci 2012; 9(3):193-199. doi:10.7150/ijms.3635. 2. Alonso-González C, González A, Menéndez-Menéndez J, Martínez-Campa C, Cos S. Melatonin as a radio-sensitizer in cancer. Biomedicines. 2020;8(8):247. doi:10.3390/biomedicines8080247 3. Maggiorella L, Barouch G, Devaux C, Pottier A, et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012 Sep;8(9):1167-81. doi: 10.2217/fon.12.96. PMID: 23030491. 4. Zhang P, Marill J, Darmon A, Anesary NM, Lu B, Paris S. NBTXR3 radiotherapy-activated functionalized hafnium oxide nanoparticles show efficient antitumor effects across a large panel of human cancer models. Int J Nanomedicine. 2021;16:2761-2773. doi:10.2147/IJN.S301182 5. Yu S, Wang Y, He P, et al. Effective combinations of immunotherapy and radiotherapy for cancer treatment. Front Oncol. 2022;12:809304. doi:10.3389/fonc.2022.809304 6. Darmon A, Zhang P, Marill J, Anesary NM, Da Silva J, Paris S. Radiotherapy-activated NBTXR3 nanoparticles modulate cancer cell immunogenicity and TCR repertoire. Cancer Cell Int. 2022;22(1):208. doi:10.1186/s12935-022-02615-w 7. Fasano M, D’Onofrio I, Belfiore MP, et al. Head and neck squamous cell carcinoma in elderly patients: role of radiotherapy and chemotherapy. Cancers (Basel). 2022;14(3):472. doi:10.3390/cancers14030472 8. Bonvalot S, Rutkowski PL, Thariat J, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 2019;20(8):1148-1159. doi:10.1016/S1470-2045(19)30326-2